Delving into the Capabilities and Prospects of Enhanced Reality Gadgetry
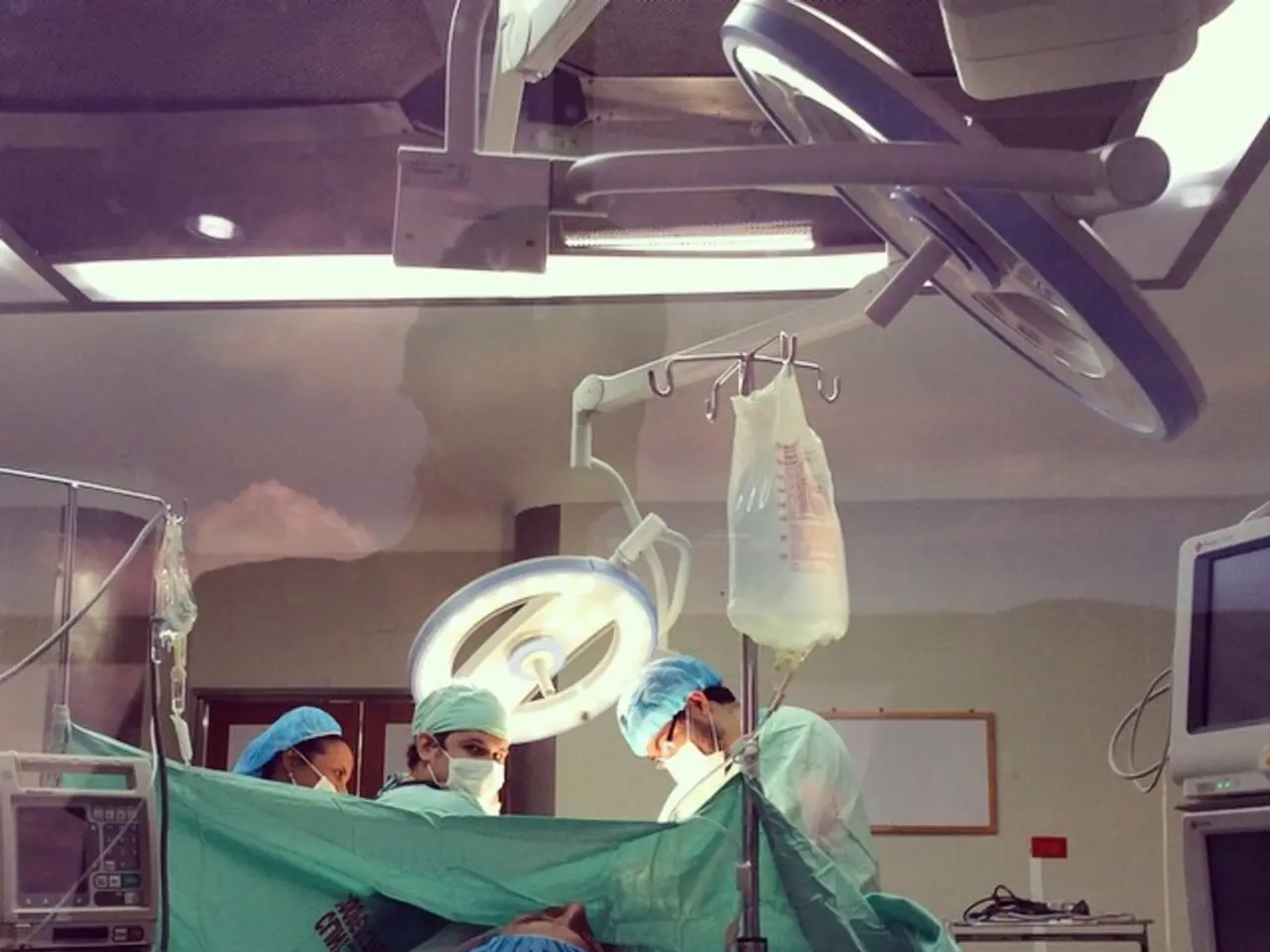
In the rapidly evolving world of medicine, augmented reality (AR) technology is making a significant impact, offering medical professionals a more comprehensive understanding and approach to treatment. From preoperative planning to surgical training, AR devices are optimising patient care and revolutionising the medical field.
However, the integration of AR technology in healthcare isn't without its challenges. Usability engineering, cybersecurity, and regulatory compliance are critical considerations to ensure safe and effective clinical integration.
### Usability Engineering: Prioritising User Needs
AR devices must cater to diverse patient and clinician needs, accommodating physical and cognitive limitations. User-centred design principles are essential, favouring interactions that avoid reliance on fine motor skills, such as eye-tracking or voice commands, to reduce physical demand.
Ergonomics and comfort are also crucial, minimising discomfort and enhancing adherence and effectiveness of AR interventions. Interfaces must be straightforward, minimising cognitive load, and facilitating easy navigation and control. Device compatibility across various AR hardware is essential for broad adoption, while advanced AR systems leveraging AI should adapt content in real time based on user physiological and emotional states to optimise therapeutic outcomes.
### Cybersecurity: Protecting Patient Data and Connectivity
Cybersecurity is a significant concern for AR devices used in medical settings, as they must not introduce threats to the patient, their privacy, or the hospital or medical institution. AR devices collecting sensitive patient data must implement stringent privacy controls and data security protocols to prevent unauthorised access, especially given the integration with mobile and networked platforms.
Medical AR devices often require reliable, high-speed connections for remote functions or AI processing. Cables, wireless links, and interfaces must comply with medical-grade standards to ensure safe, interference-free operation. Components interacting directly with patients, such as cables or wearables, must meet biocompatibility and sterilizability standards to prevent contamination and infection risks in clinical settings.
### Regulatory Compliance: Ensuring Quality and Safety
Regulatory entities must ensure that AR medical devices provide the same or better quality of care as other approved devices or treatment options. AR medical devices must fulfill stringent regulatory requirements concerning biocompatibility, electromagnetic interference (EMI) shielding, electrical safety, and sterilization to gain approval for clinical use.
Regulatory bodies require evidence from well-designed clinical trials demonstrating device safety, efficacy, and long-term outcomes. Issues such as small sample sizes, short follow-up periods, and inconsistent outcome measures must be addressed through rigorous study designs and standardised metrics. Effective regulatory compliance often demands early involvement of experts from engineering, clinical, and regulatory domains to tailor device design and testing to medical needs.
Precision is paramount in the operating room, and augmented reality devices provide surgeons with valuable tools to enhance their surgical precision. However, these devices can only be effective if they're designed, developed, and tested according to the appropriate regulatory requirements, cybersecurity standards, and usability principles.
With the industry knowledge and expertise in medical device engineering, companies like Sterling Medical Devices are bringing medical devices to market faster, ensuring things are done correctly the first time. The successful deployment of AR medical devices hinges on designing for usability across diverse users, securing patient data and device connectivity, and rigorously meeting medical regulatory standards through collaborative, evidence-based development processes.
- Advancements in data-and-cloud-computing technology can aid in the design and testing phase of AR medical devices, enabling efficient storage, processing, and analysis of user data to tailor AR devices according to user physiological and emotional states, enhancing treatment outcomes.
- Sterling Medical Devices, leveraging technology in medical devices, can expedite the market release of AR devices while prioritizing regulatory compliance, usability engineering, and cybersecurity, ensuring clinical integration is safe, effective, and optimised for medical professionals and patients alike.